TABLE OF CONTENTS
STAY CONNECTED
EXPLORE MORE
- Compliance (7)
- Data Room (29)
- Life Science (2)
- Mergers & Acquisition (1)
- News (1)
- Secure Collaboration (10)
- Self Provisioning (3)
- Uncategorized (1)
Introduction
Regulatory compliance plays a crucial role in the life sciences sector, and firms must make sure their paperwork meets FDA requirements to steer clear of setbacks or fines.
Govern 365’s Data Room functionality helps companies manage regulatory documentation more effectively, reducing the risk of errors, improving transparency, and speeding up the approval process.
Govern 365’s Data Room feature assists companies to handle regulatory documents by cutting down on mistakes, boosting clarity, and quickening the approval steps.
Key Benefits:
1. Centralized Document Hub
Govern 365 lets companies store all FDA and regulatory papers in one spot making it a breeze to arrange, reach, and distribute to interested parties. This centralized approach cuts down on the chance of lost or outdated files.
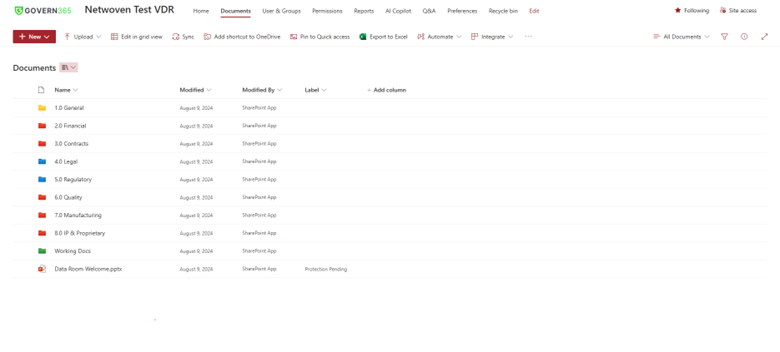
2. Regulatory Compliance Lists
Govern 365’s system can add regulatory lists helping companies make sure all needed paperwork is ready before they submit it. This lowers the odds of costly holdups due to missing or unfinished files.
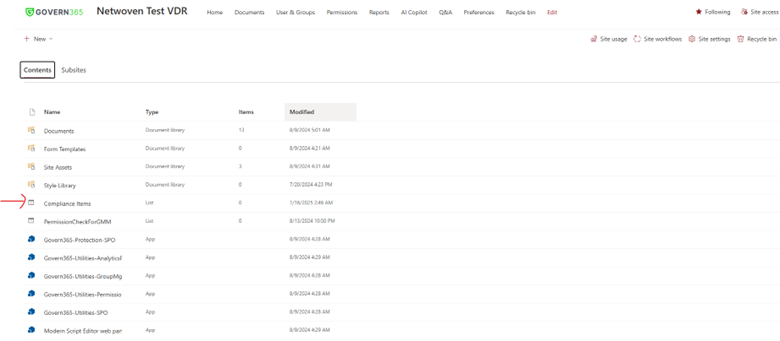
3. Audit Trails for Regulatory Reviews
Govern 365 keeps track of all document activity on its own. It records who looked at, changed, or downloaded files. This record makes it simpler to follow FDA rules. It also helps to show openness and responsibility when regulators check things over.
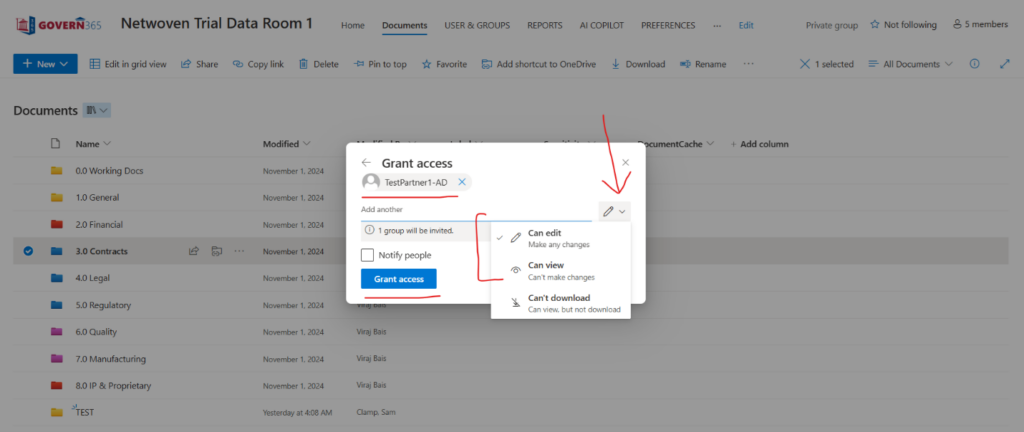
This is a view from Govern 365 app for data room owners.
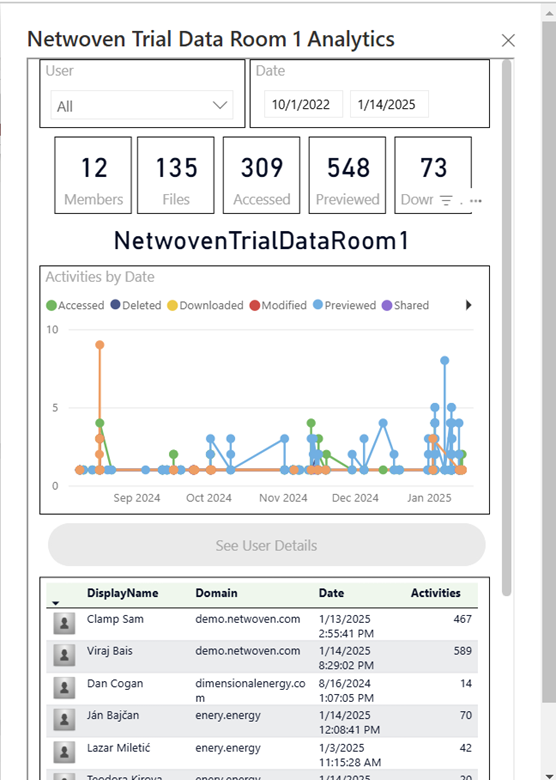
This is the full view from the actual data room.
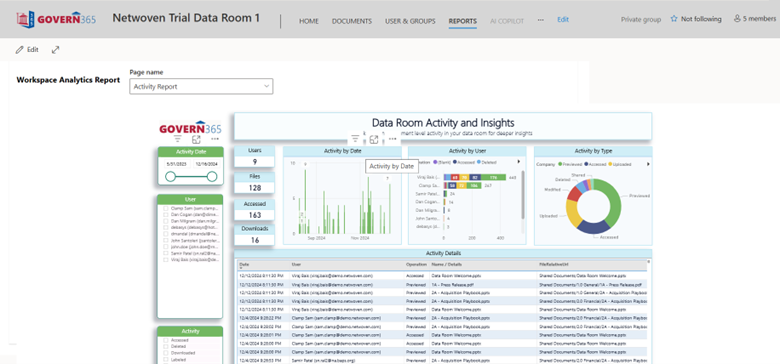
4. Secure Teamwork with Regulators
Govern 365 lets companies share files with FDA officials or regulatory advisors in a safe monitored space. Companies can give or take away access as needed, making sure the right people can see sensitive compliance files.
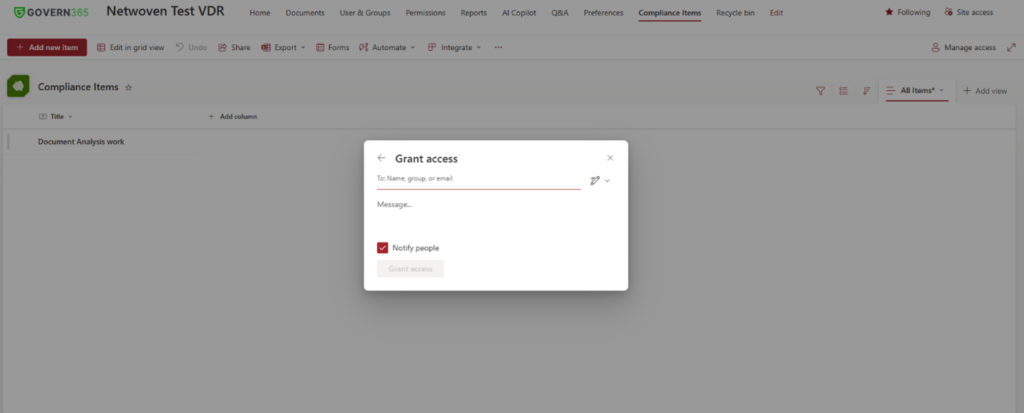
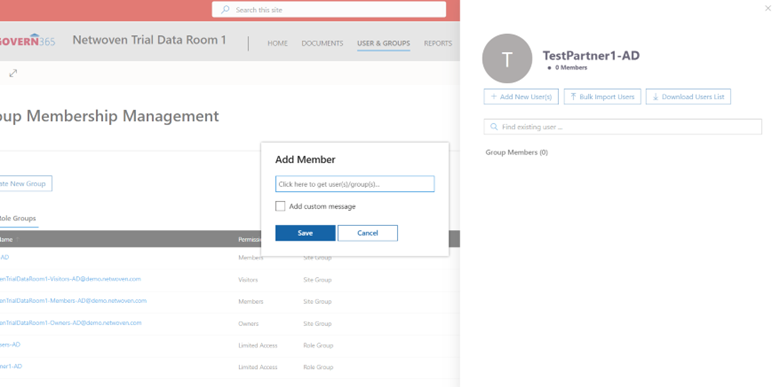
This entire teamwork happens through Group Membership Management (GMM). The below screenshot explains the same. Here, one has to create a new group.
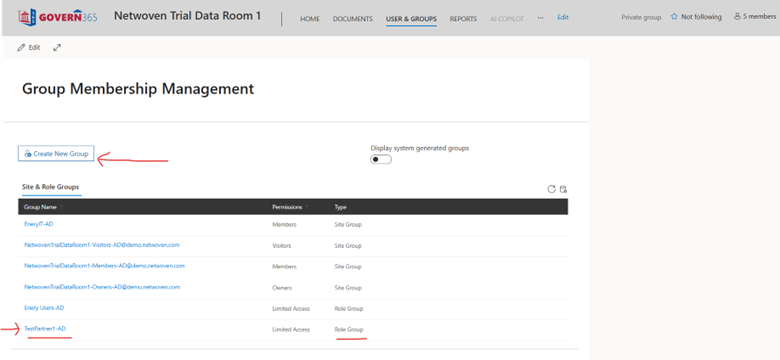
Then, one needs to manage and grant roles to users in that group.
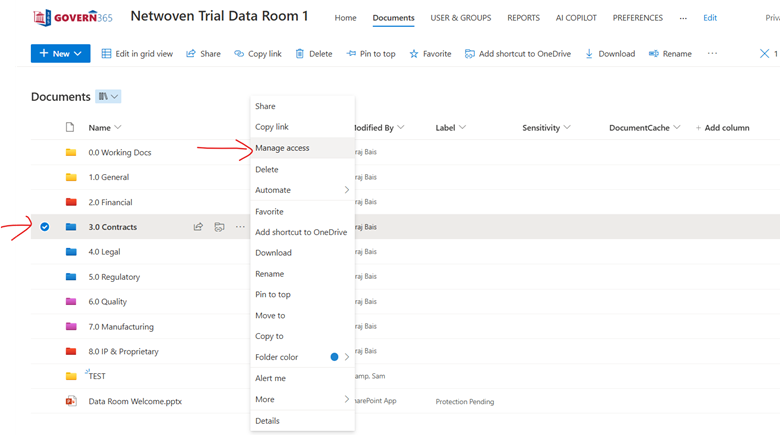
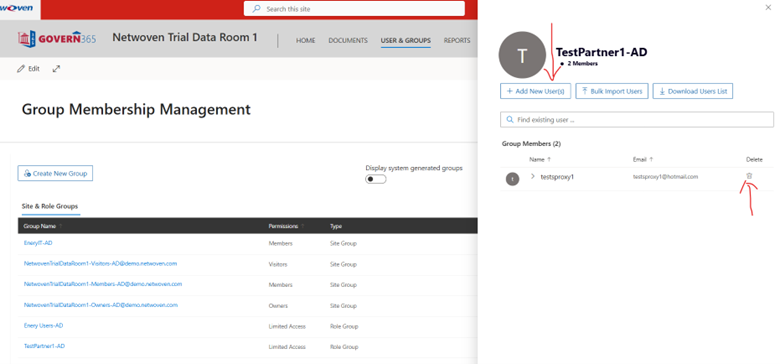
Then we recommend the data room owner to add respective people in that group. When they think access needs to be revoked, they can remove the user.
Conclusion
Govern 365 gives life sciences companies the tools to make regulatory compliance easier. By keeping FDA paperwork safe and organized, companies can lower the chance of mistakes and pave the way for a smoother journey to market. If this sounds interesting, sign up for a free demo and we will be happy to walk you through Govern 365.